2 Models:
Standard and M
Full-Leg, Knee-Length, and Arm Applications
EzLymph

Intermittent Pneumatic Compression
EzLymph M

Sequential Pneumatic Compression
(multi-chamber sequential)
Advanced Compression Therapy for Lymphedema Relief!
The EzLymph series of intermittent compression pumps and sleeves relieve discomfort and improve mobility in patients with lymphedema.
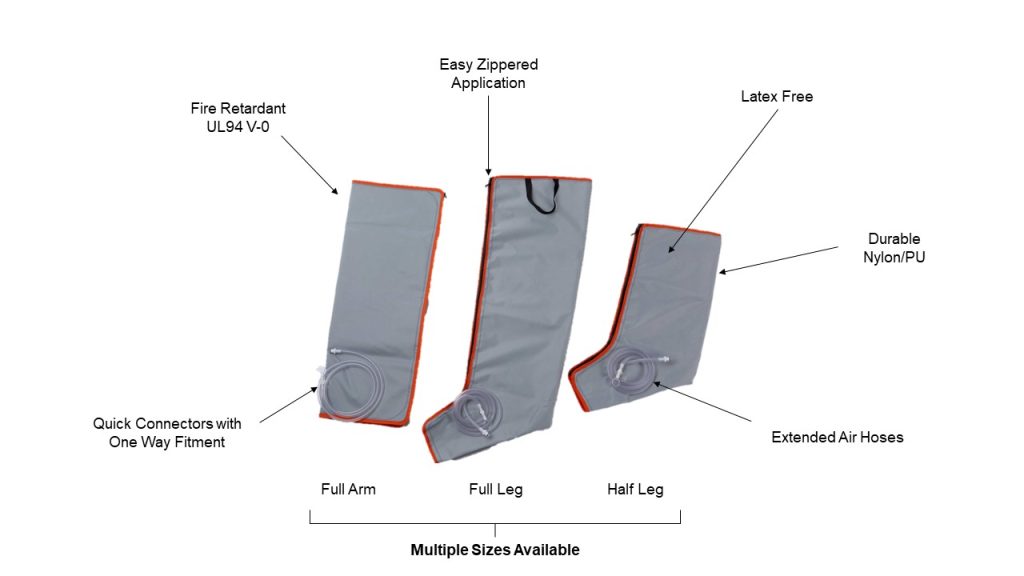
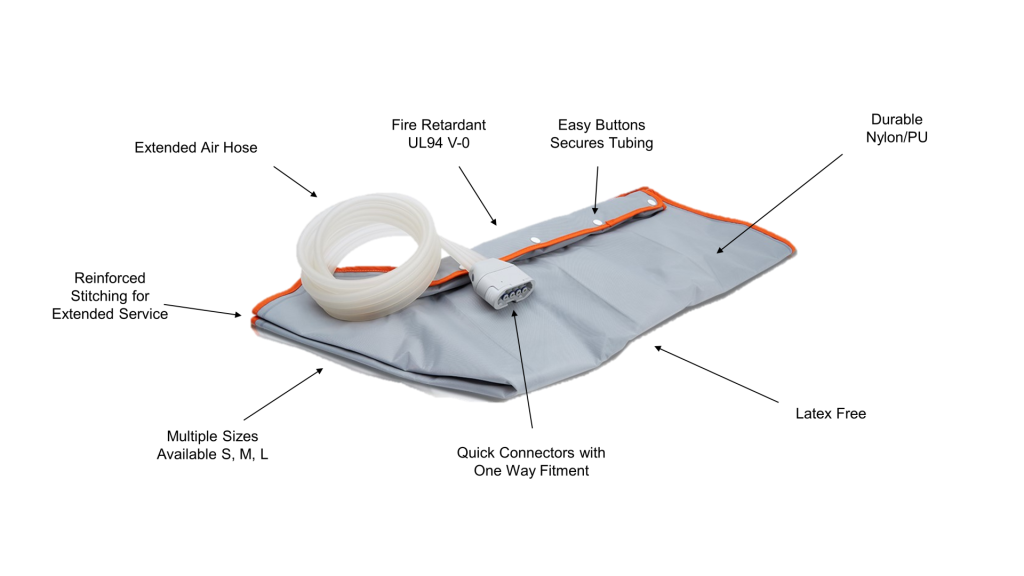
Easy Compression Sleeves for Enhanced Patient Well-being
The compression sleeves are simple to apply, and have been designed to ensure the comfort of the patient and effective therapy.
Adjustable Compression to Meet Individual Patient Needs
Single and multi-chamber configurations can be adjusted through a range of pressures to suit the needs of the patient.
Choose the Lymphedema Solution that is right for you.
Let’s chat about it click here.
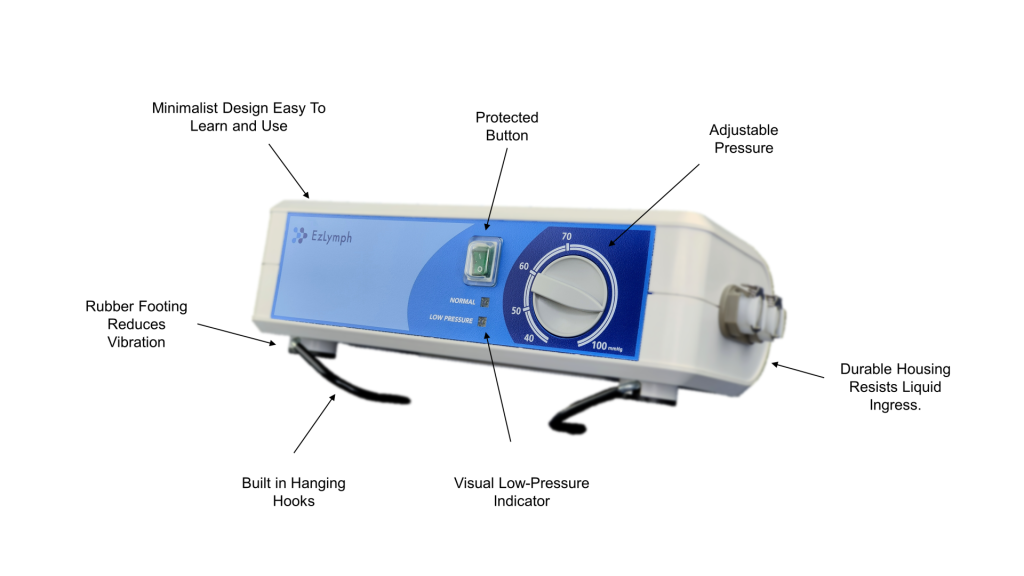
EzLymph
Specifications
| Size | 11.02” x 5.51” x 2.76” |
| Weight | 3.75 lbs |
| Display | Visual Pressure Control Dial |
| Pressure Range | Adjustable 30-90mmHg: (Gradient optional) |
| Therapy Type | Uniform or Gradient |
| Cycle | Preset 180 seconds Total Inflate: 90 seconds Deflate: 90 seconds Cycle: 180 seconds |
| IP Rating | IP21 |
| Power | AC 100-240V 50/60Hz (fused) |
s
hello. I was preevously

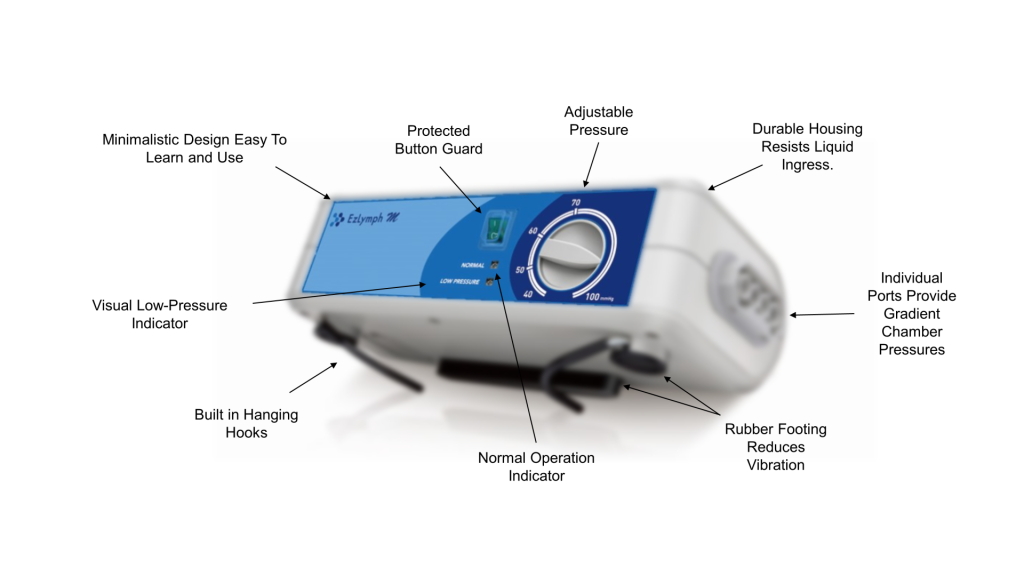
EzLymph
EzLymph M
Specifications
| Size | 11.02” x 5.51” x 2.76” |
| Weight | 3.75 lbs |
| Display | Visual Pressure Control Dial |
| Pressure Range | Adjustable 30-90mmHg |
| Therapy Type | Sequential |
| Cycle | 30 seconds inflating for each chamber Total Inflate: 120 seconds Deflate: 60 seconds Cycle: 180 seconds |
| IP Rating | IP21 |
| Power | AC 100-240V 50/60Hz (fused) |
hello. I was preevously chatting with Merly Diaz before we could get a resolution. I just sent an email to her at merly.diaz@ionos.com. Context of the issue, not including the attachments is:

EzLymph M
All our products are FDA Cleared and CE Marked and also meet the key quality standards:
ISO 13485, ISO 14971, and IEC 60601-1.
We put our patients first: our designs and materials are built to streamline the experience of caregivers while improving the comfort, safety, and effectiveness for end users.